|
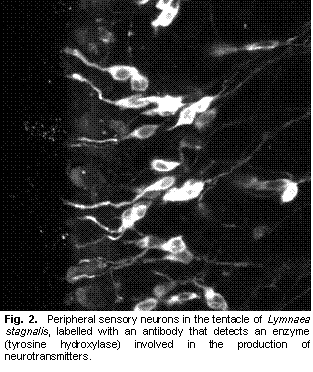
Gastropod molluscs have provided insight into many neuroethological problems. A combination of easily described behaviours, relatively simple nervous systems, re-identifiable neurons, and experimentally amenable preparations, has promoted work on many aspects of the neural control of behaviour. Studies of gastropods have focused on various aspects of neuroethology, including environmental sensory cues that guide behaviours, sensory systems, central processing and motor output that creates behaviours (Chase 2002). However, the mechanosensory system, and in particular how mechanosensory information reaches the central nervous system have not been addressed. Indeed, peripheral mechanoreceptors (those with cell bodies outside the central nervous system) are poorly understood in gastropods (Xin et al. 1995). I am studying the mechanosensory pathways that lead from the cephalic sensory organs to the central nervous system in L. stagnalis. Building on previous work (Nakamura et al. 1999), I have used several neuroanatomical techniques, including backfills, vital staining, and immunohistochemistry to study the peripheral innervation of the snail lips and tentacles. These observations, in combination with previous reports, have identified several candidate sensory cell types that may be involved in either mechanosensation or chemosensation. The neurotransmitters present in these cells include, but are not limited to, catecholamines (Croll et al. 1999), histamine (Hegedus et al. 2004), and nitric oxide (Elphick et al. 1995). The next step in this research was to determine the sensory roles of the different cell types. Behavioural analysis of pharmacologically manipulated animals with either neurotransmitter synthesis inhibitors or receptor blockers can give indications of cell functions (e.g., Romanova et al. 1996). In particular, I hypothesized that the catecholaminergic cells present in L. stagnalis (Croll et al. 1999) and also found in other molluscs (Croll 2001) were mechanosensory. If so, blocking catecholamine synthesis and therefore depleting catecholamines should cause a difference in behaviours that rely on mechanosensation. I attempted to affect catecholamine synthesis by bathing the animals in a 5mM solution of L α methyl-p-tyrosine (αΜPT) (Pani and Croll 2000). However, analysis of time lapse videos of experimental and control animals showed no differences in crawling activities, obstacle encounters, or feeding. Indeed, no appreciable difference of any sort was observable between the two groups of animals. I initially chose bath applications because I hoped to have a greater effect on the peripheral catecholaminergic sensory cells rather than central catecholaminergic cells (which would confound any conclusions drawn on behavioural changes caused by the treatment). However, since neither repeated short duration baths nor continuous baths resulted in any behavioural effects, I also attempted injections with αΜPT. These also showed no appreciable behavioural differences to saline injected animals. (Tests with dyed saline suggested that injections were able to add solutions to the hemocoel, but worryingly, some animals appeared to excrete the dye within a few hours of injection.) As a third option, I tried to manipulate catecholamine levels by used the dopamine receptor blocker Haloperidol. This drug has been shown to reduce catecholamines in molluscs (Sakharov et al. 1996). Bath treatment with 1-2 μM Haloperidol did substantially affect the behaviour of some snails, drastically reducing locomotion, and possibly reducing the animal’s ability to ‘grip’ the substrate. However, the results so far have been erratic, with some animals apparently unaffected. Furthermore, tests using the FaGlu histochemical method (Furness et al. 1978), as a crude measure of catecholamines in the lips and tentacles showed no differences. Thus, at present I am unable to draw any firm conclusions on whether |

|
Fig. 1. The pond snail Lymnaea stagnalis has been used for a variety of experiments studying how the nervous system controls behaviour. |

|
or not the catecholaminergic sensory are mechanosensory. I am currently exploring several avenues for further work. First, I have discovered that L α methyl-m-tyrosine (an isomer of αΜPT) has greater pharmacological activity in molluscs (Pires,T., pers. comm.). Unfortunately, this chemical is not commercially available, but I hope to find a method to synthesize it myself and then test its effects on the snail behaviours. Second, with ambiguous behavioural results, it will be essential to use HPLC to directly measure catecholamine levels to test whether pharmacological agents affect catecholamine levels. Finally, I hope to complement these behavioural assays experiments with more direct tests of chemosensation and mechanosensation for the various putative sensory cells I have described. I plan to use optical recording methods using calcium sensitive dyes (Gelperin and Flores 1997) to directly measure the responses of the mechanoreceptors to mechanical and chemical stimuli, and thus determine the modalities of the different cell types. Over the longer term, if I am successfully able to describe gastropod mechanoreceptors, my work can then be combined with chemosensory studies (reviewed in Elliott and Susswein 2002) |
|
to extend inquiry into the integration of both senses for the control of feeding. For example, chemosensory input has been hypothesized as more important for initiation of feeding, and mechanosensation more important for maintenance of feeding (Staras et al. 1999). With data on both modalities, I will gain insight into how the nervous system is organized to create the different functional roles for the two senses. Ultimately, this research will improve our understanding of molluscan mechanosensation and neuroethology.
References Chase,R. 2002. Behavior and its neural control in gastropod molluscs. Oxford University Press, New York, NY, USA. Croll,R.P. 2001. Catecholamine-containing cells in the central nervous system and periphery of Aplysia californica. J. Comp. Neurol. 441: 91-105. Croll,R.P., Voronezhskaya,E.E., Hiripi,L., and Elekes,K. 1999. Development of catecholaminergic neurons in the pond snail, Lymnaea stagnalis: II. Postembryonic development of central and peripheral cells. J. Comp. Neurol. 404: 297-309. Elliott,C.J.H. and Susswein,A.J. 2002. Comparative neuroethology of feeding control in molluscs. J. Exp. Biol. 205: 877-896. Elphick,M.R., Kemenes,G., Staras,K., and O'Shea,M. 1995. Behavioral role for nitric-oxide in chemosensory activation of feeding in a mollusk. J. Neurosci. 15: 7653-7664. Furness,J.B., Heath,J.W., and Costa,M. 1978. Aqueous aldehyde (Faglu) methods for the fluorescence histochemical localization of catecholamines and for ultrastructural studies of central nervous tissue. Histochem. Cell Biol. 57: 285-295. Gelperin,A. and Flores,J. 1997. Vital staining from dye-coated microprobes identifies new olfactory interneurons for optical and electrical recording. J. Neurosci. Methods 72: 97-108. Hegedus,E., Kaslin,J., Hiripi,L., Kiss,T., Panula,P., and Elekes,K. 2004. Histaminergic neurons in the central and peripheral nervous system of gastropods (Helix, Lymnaea): An immunocytochemical, biochemical, and electrophysiological approach. J. Comp. Neurol. 475: 391-405. Nakamura,H., Ito,I., Kojima,S., Fujito,Y., Suzuki,H., and Ito,E. 1999. Histological characterization of lip and tentacle nerves in Lymnaea stagnalis. Neuroscience Research 33: 127-136. Pani,A.K. and Croll,R.P. 2000. Catechol concentrations in the hemolymph of the scallop, Placopecten magellanicus. Gen. Comp. Endocrinol. 118: 48-56. Romanova,E.V., Rubakhin,S.S., and Rozsa,K.S. 1996. Behavioral changes induced by GABA-receptor agonists in Lymnaea stagnalis L. Gen. Pharmacol. 27: 1067-1071. Sakharov,D.A., Voronezhskaya,E.E., Nezlin,L., Baker,M.W., Elekes,K., and Croll,R.P. 1996. Tyrosine hydroxylase-negative, dopaminergic neurons are targets for transmitter-depleting action of haloperidol in the snail brain. Cell. Mol. Neurobiol. 16: 451-461. Staras,K., Kemenes,G., and Benjamin,P.R. 1999. Electrophysiological and behavioral analysis of lip touch as a component of the food stimulus in the snail Lymnaea. J. Neurophysiol. 81: 1261-1273. Xin,Y.P., Weiss,K.R., and Kupfermann,I. 1995. Distribution in the central-nervous-system of Aplysia of afferent-fibers arising from cell-bodies located in the periphery. J. Comp. Neurol. 359: 627-643.
|