|
Some blue-ringed octopuses (Hapalochlaena spp.) contain tetrodotoxin (TTX) in their posterior salivary glands and soft tissues (Sheumack et al. 1978). This potent neurotoxin inhibits action potentials in nerve and muscle tissue (e.g. Narahasi 2001). Because TTX occurs in several independently derived taxa besides newts and octopuses, such as puffer fish, clams, ribbon worms, and frogs (Miyazawa and Noguchi, 2001), involvement of symbiotic bacteria is hypothesized (Simidu et al. 1987, Yasumoto et al. 1989). Several strains of bacteria, including Vibrio spp., cultured from pufferfish (Noguchi et al. 1987) and the blue-ringed octopus (Hapalochlaena lunulata, Hwang et al. 1989) appeared to produce TTX. Hwang et al. (1989) cultured bacteria from the posterior salivary glands (PSG) of Hapalochlaena lunulata and found trace amounts of TTX in the colonies. However, Matsumura (1995) has called for a re-evaluation of the toxin identification in bacterial studies, because the medium on which the bacteria were cultured may cause a false identification of TTX when identified by high performance liquid chromatography (HPLC) or gas-chromatography coupled with mass-spectroscopy (GC-MS). Moreover, presence of synthetic byproducts of TTX in frogs (Daly, 1995) and lack of bacteria coincident with TTX-containing tissues in newts (Lehman et al. 2004) suggest a bacterial symbiont may not explain the origin of TTX in all cases. In the octopuses examined (Hwang et al. 1989), quantities of TTX were extremely low per bacterial culture and disagree with organismal levels of TTX. Chromatographic profiles of the purported toxin in this study were poorly resolved and near the limits of equipment detection. While bacterial production of TTX may have simply been inhibited outside the octopus host, re-examnination of TTX production and distribution in blue-ringed octopuses is warranted. First I quantified TTX in octopus tissues. Previous work on various species of blue-ringed octopuses focused on TTX in the PSG (e.g. Croft and Howden, 1972; Scheumack et al., 1978; Sutherland et al., 1970)— the typical storage site for octopus venoms (see Ghiretti, 1960). While TTX appears to be concentrated in the PSG, one population from the Philippines and one population from South Australia revealed a larger absolute amount of TTX in the “other soft parts” (Hwang et al., 1989) and arms (Yotsu-Yamashita et al., 2007) of individual octopuses, respectively. I surveyed distribution of TTX in PSG, arms, and several previously unexamined tissue types of two species of blue-ringed octopuses, Hapalochlaena fasciata and H. lunulata. Three male and one female adult Hapalochlaena fasciata were collected by hand on snorkel near Manly, New South Wales, Australia. Fourteen male and 12 female adult H. lunulata were obtained via the pet trade from Bali, Indonesia. I used HPLC methods modified from my previous research (Williams et al. 2004), Hanifin et al. (1999), and Yasumoto and Michischita (1985) for identification and quantitative analysis of TTX in all four individuals of H. fasciata and five of H. lunulata. I also quantified TTX in the PSG of the remaining individuals of H. lunulata. Two other pygmy, but non-TTX containing octopuses, Octopus mercatorus and O. bocki, served as controls. Toxin identification was further verified during the microscopy work (see below). Tetrodotoxin was detected in all tissues surveyed of H. fasciata, including the PSG, arm, dorsal and ventral mantle, digestive gland, the anterior salivary gland, ovary, oviducal gland, sperm cord, nephridia, brachial hearts, and gills. The PSG, mantle tissue, and ink were the only tissues that registered the presence of TTX in H. lunulata. The highest concentration of TTX was found in the PSG of both species; however, the integument contained the greatest absolute amounts of TTX. Minimum total amounts of TTX per octopus ranged from 0–174 µg in H. lunulata and from 60–405 µg in H. fasciata. Additional analyses of PSG of H. lunulata revealed more TTX in the glands of males than females (Wilcoxon Mann-Whitney Test, Z = -3.545, p < 0.001). However, because these animals originated in the pet trade, unknown localities and collection dates may confound the apparent difference in toxicity between sexes. If veracity of the trend is demonstrated, this difference may be due to females allocating TTX resources to offspring rather than their own defense. Scheumack et al. (1984) extracted a TTX-like compound from the eggs of “H. maculosa,” and my own work has confirmed the presence of TTX in eggs and paralarvae or hatchlings of both H. lunulata and H. fasciata (unpubl. data). Thus, females may invest their offspring with TTX or inoculate them with TTX-producing symbiotic bacteria. If female toxicity in PSG is indeed lower than males, this implies a cost of either producing TTX or hosting TTX-producing bacteria. Another clue to the origin of TTX in blue-ringed octopuses may lie in their size. If production of TTX was endogenous to octopuses one might expect larger octopuses capable of producing more toxin resources. Toxicity of PSG toxicity serves as a useful proxy for total octopus toxicity because the amount of TTX in PSG correlated well with estimates of minimum total amount of TTX in whole octopuses (Spearman’s Rank Correlation, ρ = 0.767, p = 0.016). Toxicity of the PSG (and thus total octopus toxicity) in H. lunulata does not correlate with octopus size (Spearman’s Rank Correlation, ρ = -0.060, p = 0.775). While not definitive, this lack of relationship is consistent with the hypothesis of TTX-producing symbionts. During the second phase of this research I examined distribution of TTX using immuno-labeling and microscopic techniques. Tissue samples of three H. fasciata and 3 H. lunulata from above were chosen for comparison. I labeled TTX with a monoclonal antibody, which was consequently bound to a secondary antibody with a fluorescent marker. This allowed me to visualize the distribution of TTX in the PSG of H. fasciata and H. lunulata and in the arm of H. lunulata. No gross morphological differentiation is apparent at the whole PSG level in blue-ringed octopuses; i.e., there is no evidence of a specialized structure that might serve as a nursery for symbiotic bacteria. Posterior salivary glands in blue-ringed octopuses differ from other octopuses by excreting their product via muscular contractions rather than ciliary action (Gibbs and Greenway, 1978). This could provide faster and somatic delivery of the gland constituents, including TTX. The gland is composed of dichotomously branching salivary ducts that further divide into smaller secretory ducts and terminate in closed tubules (Gibbs and Greenway, 1978). |


|
Figure 1. Fluorescent light microscope sections of posterior salivary gland (PSG) and arm of Hapalochlaena lunulata. A) PSG of H. lunulata, magnification 20X, exposure 0.200 ms, illustrating tetrodotoxin (TTX) present throughout the gland regardless of cell type. B) A section of PSG of a non-TTX containing octopus, O. bocki, magnification 40X, exposure 0.500 ms illustrating lack of fluorescence. C) Cross section of an arm-tip of H. lunulata, magnification 20X, exposure 0.200 ms, showing TTX between the integument and the central muscle (M). The integument has torn away from the muscle during sectioning in the right side of this figure. Suckers (S) are labeled for orientation. |
|
Interestingly, there is no apparent pattern to TTX distribution in Hapalochlaena PSG. Literally, the entire gland fluoresces, showing TTX fairly evenly distributed throughout the tissue (Figure 1a). In squid with light-producing symbiotic bacteria, dense colonies of bacteria form in the lumen of the adult female nidamental gland (Kaufman et al., 1998). In PSG of H. lunulata, there is neither a concentration of TTX associated with columnar/secretory cells (suggesting endogenous production of the toxin) nor concentrated fluorescence that could be readily attributed to congregations of bacteria, in the lumen or otherwise. This diffuse pattern suggests TTX may not be produced in the PSG, which could be interpreted to support an exogenous origin of the toxin. If symbionts produce TTX in another organ outside the PSG, this would explaining the lack of localized TTX fluorescence in the PSG, but this would not explain the purported bacterial producers of TTX cultured from PSG by Hwang et al. (1989). In contrast to the diffuse distribution of TTX in blue-ringed octopus PSG, a pattern appears in a cross section of an arm of one H. lunulata. Here, TTX is visible at the intersection between the integument and central muscular layer of the arm (Figure 1c). The TTX clearly visible in this section was not detectable by HPLC. |
|
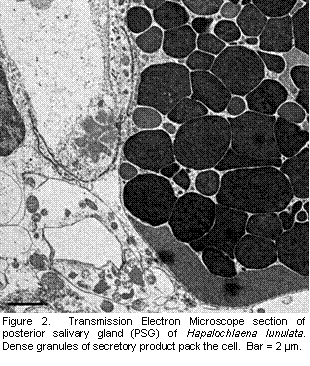
The distribution of TTX in the arm suggests sequestration of TTX in an area potentially first exposed to predators. Some octopus drop or autotomize arms as an anti-predator defense (Hanlon and Messenger, 1996; Norman, 1992). Although Hapalochlaena spp do not appear to autotomize arms, they do occasionally lose an arm to predators. A lost arm, in conjunction with a deterrent toxin, and the aposematic blue-rings of the octopus may facilitate predator learning and avoidance of these octopuses. More exploratory work with fluorescence immuno-labeling is sure to reveal additional insight into the distribution and origin of TTX in blue-ringed octopuses. In addition to the fluorescent microscopy work, I also examined PSG of H. lunulata with transmission electron microscopy (TEM) to gain a better understanding of the cellular distribution of the toxin. Theoretically, this technique allows direct visualization of bacteria. Bacteria were easily identifiable in squid nidamental gland using EM (Kaufman et al., 1998). In a survey of random sections PSG of three blue-ringed octopuses, no bacteria have been identified. Figure 2 depicts a cell packed with granules of secretory products, the location of toxin in other taxa (e.g. amphibians, Toledo and Jared, 1995, and ticks, Mans et al., |
|
2004). No bacteria associated with the granule packed cells were evident in the octopuses. Immuno-labeling of TTX with a gold marker for TEM will reveal at least the location of TTX in PSG at a finer level and granules are one logical location. However, attempts at immuno-labeling thus far in TEM have been un-successful. Immuno-staining with different gold markers and a silver enhancement are underway. In conclusion, some evidence was consistent with TTX-producing symbiotic bacteria as the ultimate origin of the toxin in blue-ringed octopuses: octopus toxicity does not correlate with octopus size and the distribution of TTX in PSG was cosmopolitan in both H. lunulata and H. fasciata. However, given the amount of TTX in each octopus, one would expect symbiotic bacteria to exist in dense colonies as they do in light producing squid. I have been unable to confirm the presence of bacterial colonies with TEM. Regardless, sequestration of TTX in between the integument and arm muscle, and the possibility of female investment of TTX into reproduction, suggest that octopuses exert some control over at least distribution if not production of TTX.
Acknowledgements I wish to thank The Malacological Society of London, The Department of Integrative Biology, University of California Berkeley (UCB), and the University of California Museum of Paleontology for funding this research. I am grateful to my co-authors on this project, Dr Roy Caldwell at UCB and Dr Michael Stark at Brigham Young University (BYU) for their contributions to this work. I also wish to thank Reena Zalpuri for her assistance in the electron microscope lab at UCB, Dr. Michael J. Stark for advice and use of his fluorescent light microscope and supplies in his laboratory at BYU, Dr David Thompson at BYU for technical assistance, and Dr. Edmund Brodie Jr. for advice and use of his laboratory, HPLC equipment, and supplies at Utah State University. Collecting permits for H. fasciata were graciously supplied by the New South Wales Fisheries Department, Australia (permit #s P05/0101-1.0 & P05/0101-2.0).
Literature Cited Croft, J.A., Howden, M.E.H., 1972. Chemistry of Maculotxin, a potent neurotoxin isolated from Hapalochlaena maculosa. Toxicon 10: 645–651. Daly, J. W. 1995. The chemistry of poisons in amphibian skin. In: T. Eisner and J. Meinwald, Eds. The Chemistry of Biotic Interaction. National Academy of Science, Washington, D.C., USA pp. 17-28. Ghiretti, F., 1960. Toxicity of octopus saliva against Crustacea. Ann. New York Acad. Sci. 90: 726–741. Gibbs, P. J., Greenway, P. 1978. Histological structure of the posterior salivary glands in the blue ringed octopus Hapalochlaena maculosa Hoyle. Toxicon 16: 59–70. Hanifin, C. T., Yotsu-Yamashita, M., Yasumoto, T., Brodie, E. D., III, Brodie, E. D., Jr. 1999. Toxicity of dangerous prey: variation of tetrodotoxin levels within and among populations of the newt Taricha granulosa. J. Chem. Ecol. 25: 2161–2175. Hanlon, R. T., Messenger, J. B. 1996. Cephalopods behaviour. Cambridge University Press, Cambridge. Hwang, D. F., Arakawa, O., Saito, T., Noguchi, T., Simidu, U., Tsukamoto, K., Shida, Y., Hashimoto, K. 1989. Tetrodotoxin-producing bacteria from the blue-ringed octopus, Octopus maculosus. Mar. Biol. 100: 327–332. Kaufman, M. R., Ikeda, Y., Patton, C., Van Dykhuizen, G., Epel, D. 1998. Bacterial symbionts colonize the accessory nidamental gland of the squid Loligo opalescens via horizontal transmission. Biol. Bull. 194: 36–43. Lehman, E. M., Brodie, Jr., E. D., Brodie, E. D., III. 2004. No evidence for an endosymbiotic bacterial origin of tetrodotoxin in the newt Taricha granulosa. Toxicon 44: 243–249. Mans, B. J., Venter, J. D., Coons, L. B., Louw, A. I., Neitz, A. W. H. 2004. A reassessment of argasid tick salivary gland ultrastructure from an immuno-cytochemical perspective. Exp. App. Acarology 33:1 19–129. Matsumura, K., 1995. Re-examination of tetrodotoxin production by bacteria. App. Environ. Microbiol. 61: 3468–3470. Miyazawa, K., Noguchi, T. 2001. Distribution and origin of tetrodotoxin. J. Toxicol. Toxicon Rev. 20: 11–33. Narahasi, T. 2001. Pharmacology of tetrodotoxin. J. Toxicol. Toxicon Rev.20: 67–84. Noguchi, T., Hwang, D. F., Arakawa, O., Sugita, H., Deguchi, Y., Shida, Y., Hashimoto, K. 1987. Vibrio alginolyticus, a tetrodotoxin-producing bacterium in the intestines of the fish Fugu vermicularis vermicularis. Mar. Biol. 94: 625–630. Norman, M. D. 1992. Amerloctopus litoris, gen. et sp. nov. (Cephalopoda: Octopodidae), a new shallow-water octopus from tropical Austalian waters. Invert. Taxonomy 6: 567–582. Sheumack, D. D., Howden, M. E. H., Spence, I. 1978. Maculotoxin: a neurotoxin from the glands of the octopus, Hapalochlaena maculosa identified as tetrodotoxin. Science 199: 188–189. Sheumack, D. D., Howden, M. E., Spence, I. 1984. Occurrence of a tetrodotoxin-like compound in the eggs of the venomous blue-ringed octopus (Hapalochlaena maculosa). Toxicon 22: 811–812. Simidu, U., Noguchi, T., Hwang, D. F., Shida, Y, Hashimoto, K., 1987. Marine bacteria which produce tetrodotoxin. App. Environ. Microbiol. 53: 1714–1715. Sutherland, S. K., Broad, A. I., Lane, W. R. 1970. Octopus neurotoxins: low molecular weight non-immunogenic toxins present in the saliva of the blue-ringed octopus. Toxicon 8: 249–250. Toledo, R. C., Jared, C. 1995. Cutaneous granular glands and amphibian venoms. Comp. Biochem. Phys. A. 111: 1–29. Williams, B. L., Brodie, E. D., Jr., Brodie, E. D., III. 2004. A resistant predator and its toxic prey: persistence of newt toxin leads to poisonous (not venomous) snakes. J. Chem. Ecol. 30: 1901–1919. Yasumoto, T., Michishita, T. 1985. Fluorometric determination of tetrodotoxin by High Performance Liquid Chromatography. Agric. Biol. Chem. 49: 3077-3080. Yotsu, M., Endo, A., Yasumoto, T. 1989. An improved tetrodotoxin analyzer. Agric. Biol. Chem. 53: 893–895. Yotsu-Yamashita, M., Mebs, D., Flachsenberger, W. 2007. Distribution of tetrodotoxin in the body of the blue-ringed octopus (Hapalochlaena maculosa). Toxicon 49: 410–412.
|